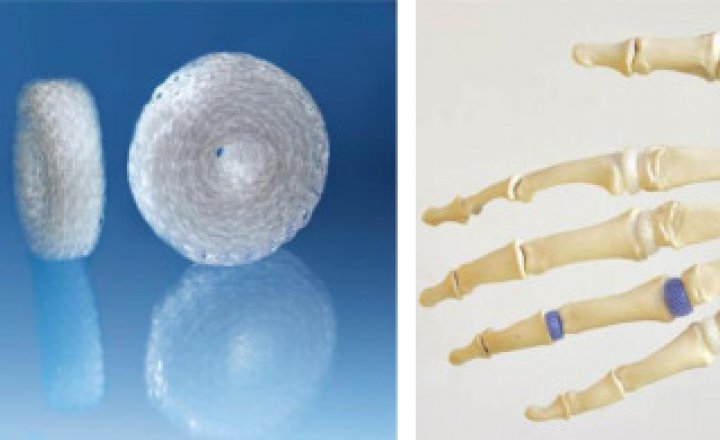
The RegJoint™ implant (Scaffdex Oy, Finland) is a bioabsorbable poly-L/D-lactide implant that acts as a temporary support in removed joint spaces. It can be used in thumb epiphyseal surgery as a spacer to prevent subsidence of the first metacarpal bone.
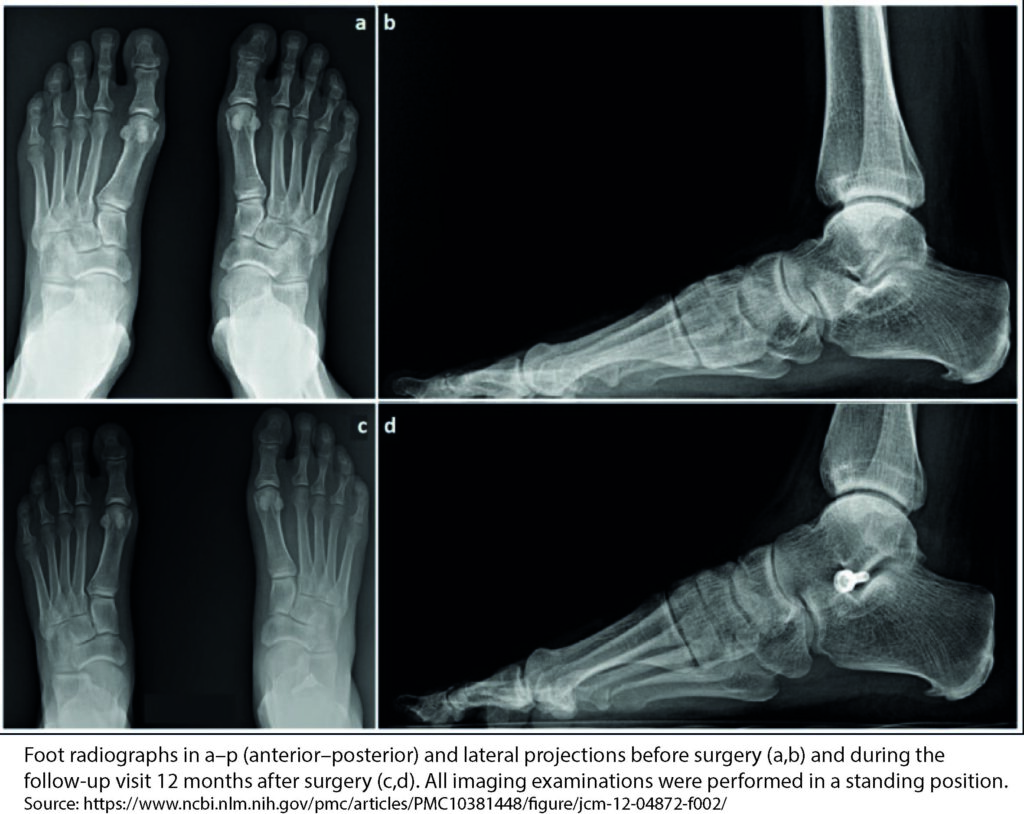
The purpose of the study presented in the publication was to evaluate the results of patients treated with this implant. Patients underwent postoperative clinical and radiological evaluation. The QuickDASH questionnaire, Patient Evaluation Measure (PEM) and a visual analog scale were used to assess pain. Among other things, grip strength, palmar and radial thumb inversion were measured. The study was conducted on twenty-two patients.
The results of the study showed that there were no postoperative wound complications. There were also no adverse soft tissue reactions or significant bone erosion. There were also no significant changes in hand function.
The study confirmed that RegJoint™ is a useful adjunct in the treatment of a select group of patients with thumb base arthritis.
The description and results of the study can be read in PubMed.